Prepare. Protect. Progress.
A burn care solution for every step of the way

Discover the Smith+Nephew burn care portfolio
Promote wound healing with our advanced burn management portfolio
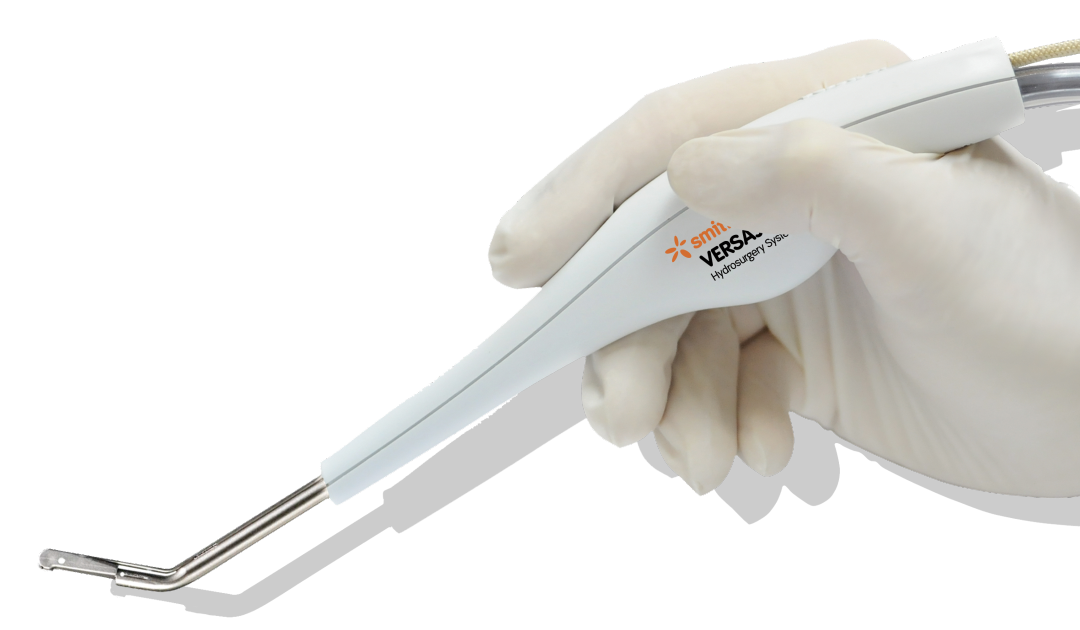
VERSAJET◊ Hydrosurgery System
ACCELERATE your surgical debridement*
- Can perform the tasks of a scalpel and suction in one indispensable tool.1
- Powerful, adjustable, pressurized sterile stream enables precise excision of necrosis and other unwanted material while preserving viable tissue for better wound bed preparation.3,4,8
- Debrides majority of wound types, including acute, chronic, and burns, with a demonstrated reduction in bacterial load.5,7
- Several studies have demonstrated that the use of the VERSAJET System may reduce inpatient hospitalization by as many as 3-6 days for chronic wounds, potentially reducing overall wound management costs.12
Indicated for partial-thickness burns.
*Compared to standard of care

SANTYL◊ Ointment
The depth of a burn is not static
- Can be applied immediately after initial burn stabilization treatment without harming healthy tissue.10,11
- Changes the burn microenvironment via the MOA to fight inflammation and stimulate the migration and proliferation of fibroblasts, keratinocytes, and endothelial cells.12-15
- Enzymatic debridement with clostridial collagenase — the active ingredient in SANTYL Ointment — has been shown in an animal study to slow conversion in partial-thickness burns.16
Indicated for severe burns.
*see Important Safety Information below

ACTICOAT◊Antimicrobial
Barrier Dressings
Act with Certainty
- Kills bacteria as quickly as 30 minutes (in vitro).17-20
- Effective against Gram-positive and Gram-negative bacteria, yeast, and fungi, including antibiotic-resistant organisms such as MRSA, VRE, and Enterobacteriaceae strains containing NDM-1 carbapenemases.21
- Provides up to 3-7* days of sustained action,23-28 allowing for fewer dressing changes and reducing stress and trauma. Limiting disturbance to the wound can also lead to faster healing.29
- Minimizes the risk of infection in partial- and full-thickness burn wounds.30-33
- Achieves an overall reduction of total treatment costs and length of hospitalization as part of an infection management protocol in burn wounds compared to standard of care.34-36
- Fewer surgical procedures such as debridement/grafts were needed when using ACTICOAT Dressings in burn wound management protocols.†30,37
- Achieved significantly faster re-epithelialization.††38
- In pediatric patients, it helps minimize infection, achieve faster re-epithelialization‡, and significantly reduce grafting procedures.†30
- Low adherent and has been shown to be less painful on removal than SSD preparations in burns.39
Indicated for first- and second-degree burns.** Can be used in both adult and pediatric populations.
*wear time is anywhere from 3 to 7 days depending on the version of ACTICOAT being used.
†compared to Silverzine™ and SSD
‡compared to Mepilex™ Ag, SSD
††compared to Mepilex™ Ag, SSD, Silverzine™, antimicrobial solution (neomycin and polymyxin)
**For full list of indications, please refer to the IFUs.

BIOBRANE◊ Temporary Biosynthetic Wound Dressing
A highly comfortable biosynthetic wound dressing
- A flexible easy to apply composite dressing with adherent properties that conforms to surface irregularities.43,44-48
- Accelerates the speed of healing and epithelialization, while reducing the need for patients to require a skin graft.49-52
- Reduces the healing time†, which‑may lead to‑a quicker discharge.49-51,54
- It’s transparent, allowing direct wound inspection.44,46,53
- Minimizes pain, after application of BIOBRANE Dressing patients experienced significant improvement in pain levels‡.49,50,55
- Provides comfort and mobility for patients using the product.44,46
- Available in a range of dressing and glove sizes.
Indicated for covering partial-thickness burns and split-thickness donor sites. Can be used in both adult and pediatric populations.

OASIS® Burn Matrix
Evidence-based solution for healing
- Good aesthetic outcomes with no complications or infections.40-42
- Closure in 100% of second-degree burns in 7-14 days in two studies.41,42
- Less scarring and more mature epidermis than silver-containing hydrofiber dressing in one study.40
- Earlier granulation tissue and more mature epidermis when compared to Suprathel® in one study.42
- No need for painful daily dressing changes.42
Indicated for partial-thickness burns.
*See important safety information below




*n=37; dressing retention was 1.92x longer.
**In a pragmatic, randomized, controlled, superiority trial. p=0.001; n=359. Estimated cost savings vs standard preventive care alone; n=359.
***Compared to previous dressings. Ambispective (retrospective and prospective) observational study. 1.66 ALLEVYN LIFE Dressings vs 3.14 previous dressings (foams or gelling fibres); n=94; p<0.001). €11.46 ALLEVYN LIFE Dressings vs €27.75 previous dressings (foams or gelling fibres); n=94; p<0.001).
****n=38. Time saving based on previous dressing, estimated for 169 patients requiring ≥3 visits per week.
- Caputo WJ, Beggs DJ, DeFede JL, Simm L, Dharma H. A prospective randomised controlled clinical trial comparing hydrosurgery debridement with conventional surgical debridement in lower extremity ulcers. Int Wound J. 2008;5(2):288-294.
- Liu J, Ko JH, Secretov E, et al. Comparing the hydrosurgery system to conventional debridement techniques for the treatment of delayed healing wounds: a prospective, randomised clinical trial to investigate clinical efficacy and cost-effectiveness. Int Wound J. 2015;12(4):456-461.
- McAleer JP, Kaplan EM, Perisich G, Axman W. A Prospective Randomized Study Evaluating the Time Efficiency of the VERSAJET™ Hydrosurgery System and Traditional Wound Debridement. Poster presented at: ACFAS 2005; New Orleans, LA.
- Granick MS, Posnett J, Jacoby M, Noruthun S, Ganchi PA, Datiashvili RO. Efficacy and cost-effectiveness of a high-powered parallel waterjet for wound debridement. Wound Repair Regen. 2006;14:394-397.
- Mosti G, Maltaliano V. The debridement of chronic leg ulcers by means of a new, fluidjet-based device. Wounds. 2006;18:227-237.
- James CV, Patel M, Ilonzo N, et al. Hydrosurgical debridement use associated with decreased surgical site-related readmissions: a retrospective analysis. Wounds. 2021;33 (6):139-142.
- Rennekampff HA, Schaller HE, Wisser D, Tanenhaus M. Debridement of Burn Wounds with a Water Jet Surgical Tool. Burns. 2006;32(1):64 – 69.
- Granick M, Boykin J, Gamelli R, Schultz G, Tenenhaus M. Toward a common language: surgical wound bed preparation and debridement. Wound Repair Regen. 2006;14:S1-S10.
- Vanwijck R, Kaba L, Boland S, Gonzales y Azero M, Delange A, Tourbach S. Immediate skin grafting of sub-acute and chronic wounds debrided by hydrosurgery. J Plast Reconstr Aesthet Surg. 2010;63:544-549.
- Shi L, Carson D. Collagenase Santyl ointment: a selective agent for wound debridement. J Wound Ostomy Continence Nurs. 2009;36(6 Suppl):S12-S16. doi:10.1097/WON.0b013e3181bfdd1a.
- Collagenase SANTYL Ointment [prescribing information]. Fort Worth, TX: Smith & Nephew, Inc.; 2018.
- Herman, I. Stimulation of human keratinocyte migration and proliferation in vitro: insights into the cellular responses to injury and wound healing. Wounds. 1996; 8:33-40.
- Riley et al. Collagenase promotes the cellular responses to injury and wound healing in vivo. J Burns Wounds. 2005; 4:112-124.
- Shi et al. Degradation of human collagen isoforms by Clostridium collagenase and the effects of degradation products on cell migration. Int Wound J. 2010; 7: 87-95.
- Sheets AR, Demidova-Rice TN, Shi L, Ronfard V, Grover KV, Herman IM (2016) Identification and Characterization of Novel Matrix-Derived Bioactive Peptides: A Role for Collagenase from Santyl® Ointment in Post-Debridement Wound Healing? PLoS ONE 11(7): e0159598.
- Frederick RE, Bearden R, Jovanovic A, Jacobson N, Sood R, Dhall S. Clostridium Collagenase Impact on Zone of Stasis Stabilization and Transition to Healthy Tissue in Burns. Int J Mol Sci. 2021;22(16):8643. Published 2021 Aug 11. doi:10.3390/ijms22168643.
- Wright JB et al. (1998) American Journal of Infection Control; 26(6): 572-577.
- Wright JB et al. (1999) Am J Infect Control ;27:344-350.
- Driffield, K; ACTICOAT Flex 3 has antimicrobial activity in 30 minutes, Data on file 0810018, Smith & Nephew.
- Driffield, K; ACTICOAT Flex 7 has antimicrobial activity in 30 minutes, Data on file 0810014, Smith & Nephew.
- 121. Daubney, L: Silver Release Testing of ACTICOAT Flex 3 Dressings. Report reference DS/08/078/R2.
- Westaim Report ref #971030 The Antimicrobial Activity of Westaim’s Acticoat Silver Coated Dressing against Clinically Relevent Organisims over an Extended Period of time.
- Driffield, K; Antimicrobial Activity of ACTICOAT Flex 3 against a Broad Spectrum of Wound Pathogens, Data on File reference 0810016.
- Newton (2010) Reducing MRSA bacteraemias associated with wounds. Wounds uk, Vol 6, No 1.
- Selcuk (2012) Comparison of the antibacterial effect of silver. Burns 38: 1204-1209.
- Sibbald et al (2001) A screening evaluation of an ionised nanocrystalline silver dressing in chronic wound care. Ostomy Wound management 47 (10), 38-4.
- Chopra (2007) Increasing use of silver-based products. Jnl. Anti Chem.
- Strohal, R. et al. Nanocrystalline silver dressings as an efficient anti-MRSA barrier: a new solution to an increasing problem. J. Hosp. Infect. 60, 226–30 (2005).
- Upton, D. and Solowiej, K. (2010). Pain and Stress as Contributors to Delayed Wound Healing. Journal of the Australian Wound Management Association, 2010;18: 3.
- Cuttle L, Naidu S, Mill J, Hoskins W, Das K, Kimble RM. A retrospective cohort study of Acticoat versus Silvazine in a paediatric population. Burns. 2007;33(6):701 – 707.
- Gago M, Garcia F, Gaztelu V, Verdu J, Lopez P, Nolasco A. A comparison of three silver-containing dressings in the treatment of infected, chronic wounds. Wounds. 2008;20(10):273 – 278.
- Huang Y, Li X, Liao Z, et al. A randomized comparative trial between Acticoat and SD-Ag in the treatment of residual burn wounds, including safety analysis. Burns. 2007;33(2):161 – 166.
- Gravante G, Caruso, R., Sorge, R., Nicoli, F., Gentile, P. and Cervelli, V. Nanocrystalline silver: a systematic review of randomized trials conducted on burned patients and an evidence-based assessment of potential advantages over older silver formulations. Annals of plastic surgery. 2009;63:201-205.
- Tonkin C, Wood F. Nanocrystalline silver reduces the need for antibiotic therapy in burn wounds. Primary Intention. 2005;13(4):163 – 168.
- Strand O, San Miguel L, Rowan S, Sahlqvist A. Retrospective comparison of two years in a paediatric burns unit, with and without ACTICOAT as a standard dressing. Ann Burns Fire Disasters. 2010;23(4):182 – 185.
- Fong J, Wood F, Fowler B. A silver coated dressing reduces the incidence of early burn wound cellulitis and associated costs of inpatient treatment: Comparative patient care audits. Burns. 2005;31(5):562 – 567.
- Peters DA, Verchere C. Healing at home: Comparing cohorts of children with medium-sized burns treated as outpatients with in-hospital applied ACTICOAT to those children treated as inpatients with silver sulfadiazine. J Burn Care & Research. 2006;27(2):198 – 201.
- Demling RH, DeSanti L. The rate of re-epithelialization across meshed skin grafts is increased with exposure to silver. Burns. 2002;28(3):264 – 266.
- Muangman P, Chuntrasakul C, Silthram S, et al. Comparison of Efficacy of 1% Silver Sulfadiazine and Acticoat for Treatment of Partial-Thickness Burn Wounds. J Med Assoc Thai. 2006;89(7):953 – 958.
- Salgado RM, Bravo L, García M, Melchor JM, Krötzsch E. Histomorphometric analysis of early epithelialization and dermal changes in mid partial-thickness burn wounds in humans treated with porcine small intestinal submucosa and silver-containing hydrofiber. J Burn Care Res. 2014;35(5):e330-e337.
- Cuenca-Pardo J, Peralta-Conde D. Quemaduras en cara ratada con escision temprana y cubiertas con matrix acelular (Face burns treated with early excision and covered with an acellular matrix). Cir Plast. 2011;21(1):11-19.
- Glik J, Kawecki M, Kitala D, et al. A new option for definitive burn wound closure – pair matching type of retrospective case-control study of hand burns in the hospitalised patients group in the Dr Stanislaw Sakiel Centre for Burn Treatment between 2009 and 2015. Int Wound J. 2017;14:849-855.
- Erdman D, Hussman J, Kucan JO. Burns. 1996;22(2):141–146Res. 2014;14(6):5–12
- Farroha A, Frew Q, El-Muttardi N, Philp B, Dziewulski P. Ann Burns Fire Disasters. 2013;XXVI(2):94–97.
- Fan C, Pek CH, Por YC, Lim GJS. Singapore Med J. 2018;59(7):360–365.
- Lesher AP, Curry RH, Evans J, et al. J Pediatr Surg. 2011;46(9):1759–1763.
- Austin RE, Merchant N, Shahrokhi S, Jeschke MG. Burns. 2015;41(4):749–753.
- Yang_J-Y, Tsai Y-C, Noordho_ MS. Burns.1989;15(3):197–203.
- Barret JP, Dziewulski P, Ramzy PI, Wolf SE, Desai MH, Herndon DN. Plastic and Reconstructive Surgery. 2000;105(1):62–65. Gerding RL, Imbembo AL, Fratianne RB. J Trauma. 1988;28(8):1265–1269.
- Gerding RL, Imbembo AL, Fratianne RB. J Trauma. 1988;28(8):1265–1269.
- Wasiak J, Cleland H, Campbell F. Cochrane Database Syst Rev. 2008(4).
- Kumar RJ, Kimble RM, Boots_R, Pegg SP. ANZ J Surg. 2004;74(8):622-626.
- Lang EM, Eiberg CA, Brandis M, Stark GB. Ann Plast Surg. 2005;55(5):485–489.
- Lal S, Barrow RE, Wolf SE, et al. Shock (Augusta, Ga). 2000;14(3):314–318; 318–319.
- Gerding RL, Emerman CL, E_ ron D, et al. Ann Emerg Med. 1990;19(2):121–124.
For detailed product information, including indications for use, contraindications, warnings, and precautions, please consult each product’s Instructions for Use (IFU) prior to use. Products may not be available in all markets because product availability is subject to the regulatory and/or medical practices in individual markets. Please contact your Smith+Nephew representative or distributor if you have questions about the availability of Smith+Nephew products in your area.
Important Safety Information
Indications: Collagenase SANTYL Ointment (“SANTYL”) is a prescription-only medication indicated for debriding chronic dermal ulcers and severely burned areas. Contraindications: SANTYL is contraindicated in patients who have shown local or systemic hypersensitivity to collagenase. Warning and Precautions: The optimal pH range of collagenase is 6 to 8. Higher or lower pH conditions will decrease the enzyme’s activity and appropriate precautions should be taken. The enzymatic activity is also adversely affected by certain detergents, and heavy metal ions such as mercury and silver which are used in some antiseptics. As such, the wound should be properly cleansed prior to application of SANTYL. Debilitated patients should be closely monitored for systemic bacterial infections because of the theoretical possibility that debriding enzymes may increase the risk of bacteremia. A slight transient erythema has been noted occasionally in the surrounding tissue, particularly when SANTYL was not confined to the wound. SANTYL is not indicated for wound closure. Discontinue use of SANTYL after granulation tissue is well-established. Adverse Reactions: No allergic sensitivity or toxic reactions have been noted in clinical use when used as directed. The risk information provided herein is not comprehensive. For complete prescribing information, please refer to the accompanying PI or visit: https://sn-burn.com/wp-content/uploads/2023/12/SAPE72-38468_SANTYL-PI-2018-FINAL-APPROVED.pdf You are encouraged to report negative side effects of prescription drugs to FDA. Visit MedWatch or call 1-800-FDA-1088.
For OASIS: This device is derived from a porcine source and should not be used in patients with known sensitivity to porcine materials. This device is not indicated for use in third degree burns.